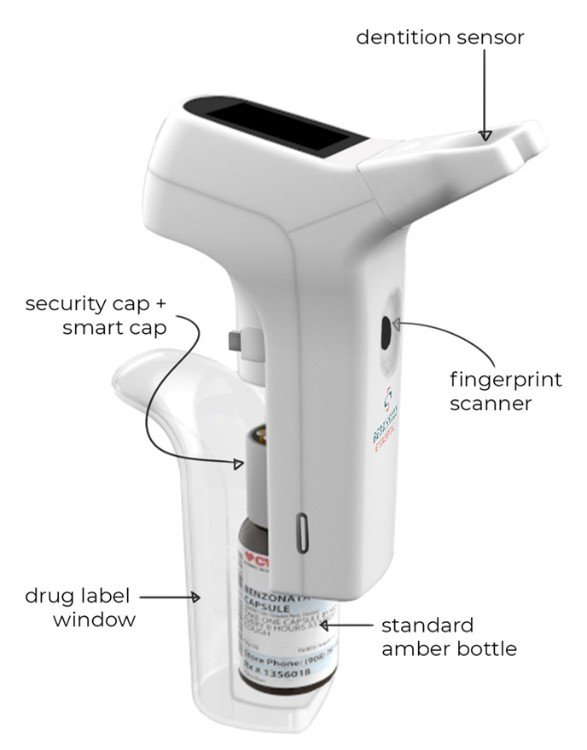
Berkshire Biomedical’s COPA™ System represents a transformative innovation in the field of opioid use disorder (OUD) treatment, offering a secure and precise method for take-home methadone medication management. At the heart of this technology lies its ability to deliver oral liquid medications only to the Authenticated Intended User (AIU™) through dual biometric verification—fingerprint and dentition. This level of authentication ensures that methadone is administered safely and exclusively to the intended patient, reducing risks of diversion, misuse, or accidental ingestion. The system is designed to support remote monitoring, offering clinicians real-time insight into dosing adherence, and enhancing overall treatment accountability.
The importance of this innovation is underscored by a recently awarded Fast-Track Small Business Innovation Research (SBIR) grant from the National Institute on Drug Abuse (NIDA), part of the National Institutes of Health (NIH). The grant—valued at approximately $2.9 million over 30 months—will support Phase I and Phase II clinical studies aimed at evaluating patient retention and therapeutic outcomes associated with COPA-based take-home methadone therapy. As daily travel to Opioid Treatment Programs (OTPs) has been shown to negatively impact treatment retention, the ability to administer methadone securely at home could be a critical advancement in removing participation barriers and improving long-term recovery rates.
The COPA System is not just a technical solution, but a patient-centered advancement that could reshape how MOUD programs are delivered. It promotes greater autonomy for stable patients, supports early access to treatment, and ensures safety in home-based medication administration. By addressing the logistical challenges and social stigma tied to daily clinic visits, the system could lead to broader program adoption, higher retention rates, and improved health outcomes. With NIH support validating its potential, COPA stands poised to become a vital tool in the national strategy to combat the opioid crisis.