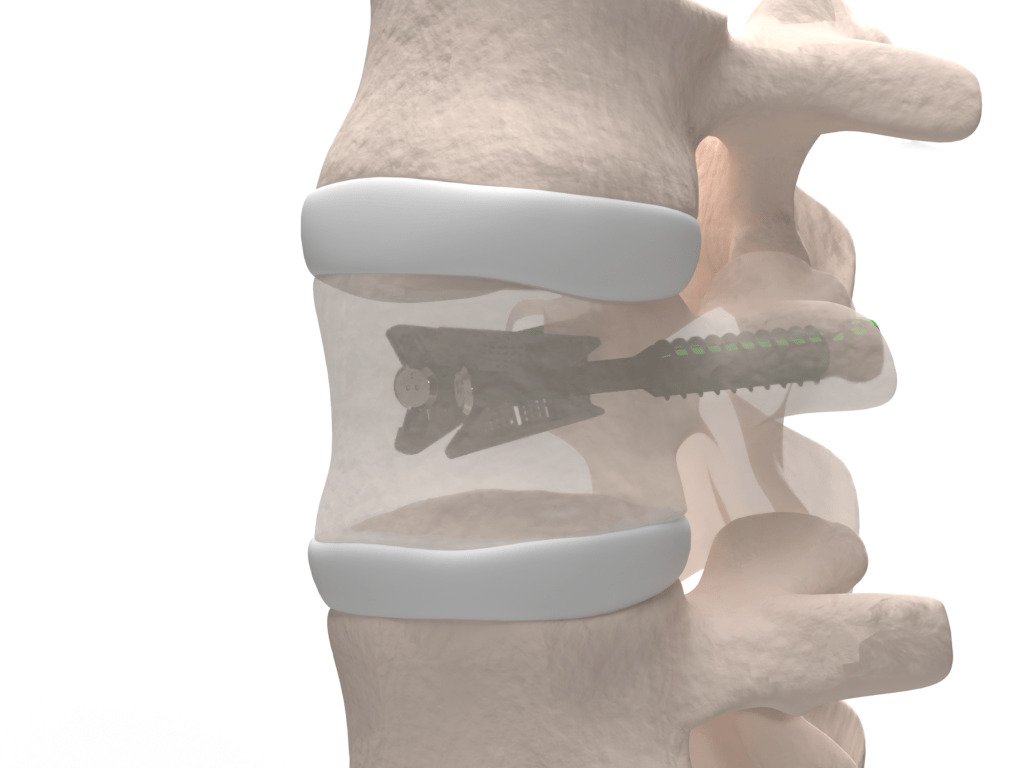
Amber Implants’ VCFix® Spinal System represents a significant innovation in the treatment of vertebral compression fractures (VCFs), a condition that affects millions globally and often leads to debilitating pain and limited mobility. Unlike traditional approaches that rely heavily on the injection of bone cement, VCFix® offers a more advanced, versatile alternative that can be used with or without cement and without requiring posterior fixation. Its unique mechanical design supports spinal stability while minimizing the risk of complications commonly associated with current procedures, such as cement leakage and adjacent vertebral fractures.
The benefits of VCFix® were clearly demonstrated in the first-in-human clinical trial, where all enrolled patients completed one-year follow-up evaluations. The data revealed significant improvements in pain relief and functional recovery. Patients reported an average decrease of over 8 points on the pain scale within six months, with benefits maintained throughout the year. Similarly, disability scores saw a marked reduction, with an 84-point improvement noted at the six-month mark. Importantly, these positive outcomes were achieved without any device-related adverse events, underscoring the safety and efficacy of the system.
This breakthrough has substantial clinical and commercial implications. With over 8.6 million vertebral fractures occurring each year and the treatment market expected to surpass $2.5 billion by 2028, there is a pressing need for safer, more adaptable solutions like VCFix®. The system’s FDA Breakthrough Device Designation and its pending pivotal multi-site trial in Europe position Amber Implants to redefine the standard of care for spinal trauma. These one-year clinical results not only validate the device's design and functionality but also open the door for broader adoption in both osteoporotic and traumatic fracture cases.