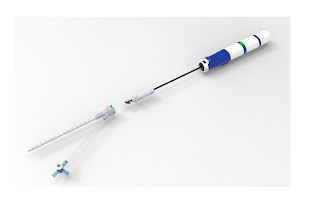
Vivasure Medical’s PerQseal® Elite represents a significant advancement in the field of large-bore vascular closure, offering the world’s first fully bioabsorbable and sutureless closure system for both arterial and venous access. The recent PMA submission to the U.S. FDA, along with the expanded CE mark approval in Europe, underscores the company’s commitment to redefining vascular access management with a next-generation solution. Built on Vivasure’s proprietary PerQseal platform, the Elite system is engineered to eliminate the need for permanent implants, leaving the vessel to heal naturally without foreign materials such as metal or collagen.
Designed for use following percutaneous cardiovascular procedures, including structural heart and electrophysiology interventions, PerQseal Elite simplifies deployment by allowing placement from inside the vessel—removing the need for pre-closure steps required by traditional technologies. Its fully bioresorbable patch promotes natural vessel healing, reduces the risk of long-term complications, and offers physicians a streamlined, efficient closure process. This is especially critical in complex, high-risk procedures where precise, reliable closure is essential for patient outcomes and procedural success.
With regulatory momentum building on both sides of the Atlantic, PerQseal Elite is well-positioned to address the growing global demand for minimally invasive solutions in large-bore vascular access. The PMA submission and CE mark expansion not only validate the system’s strong clinical performance, as demonstrated in the PATCH study, but also signal Vivasure’s broader goal of transforming vascular closure through innovation. As adoption of transcatheter procedures rises, PerQseal Elite sets a new benchmark for safety, simplicity, and long-term patient care.