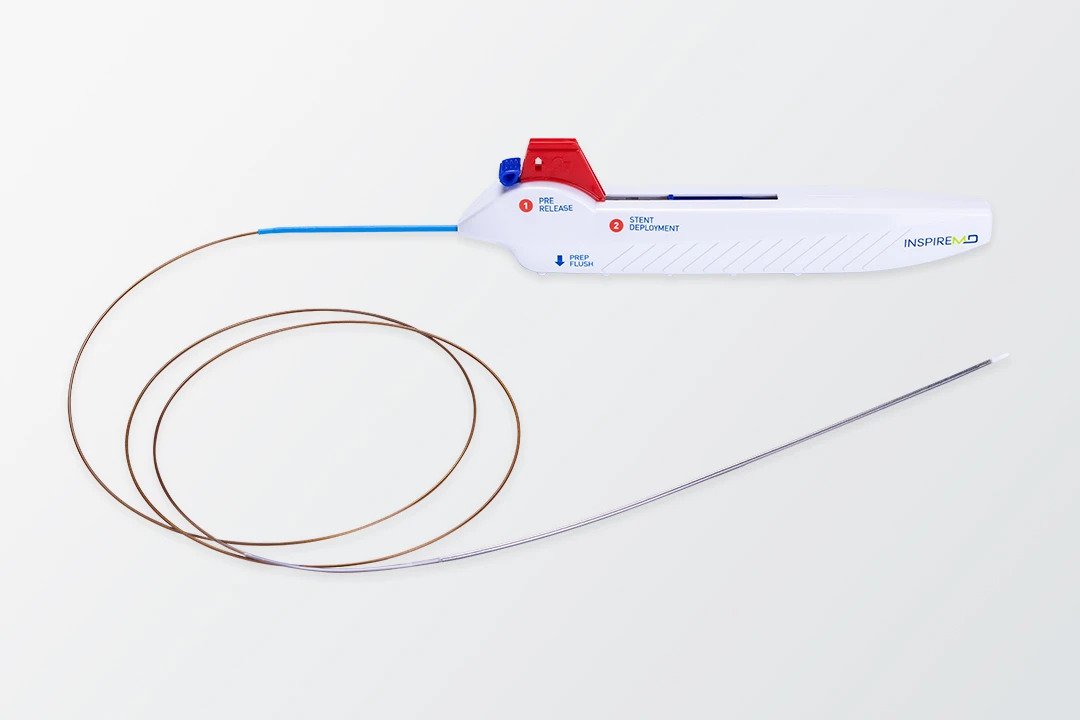
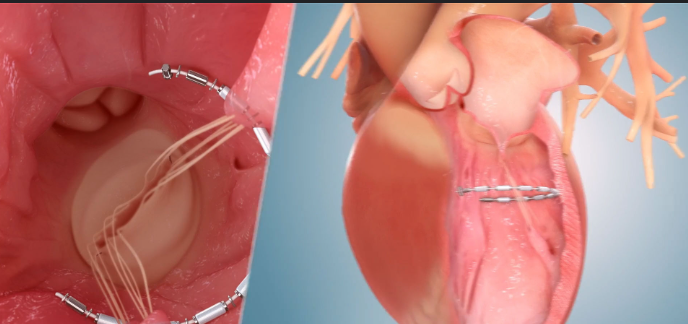
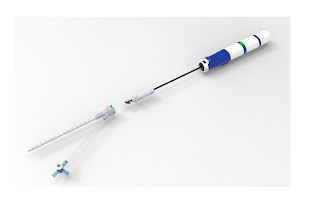
InspireMD’s CGuard® Prime Carotid Stent System represents a next-generation innovation in carotid artery disease treatment, designed to significantly reduce the risk of stroke. At the heart of this technology is the MicroNet™ mesh—a unique, proprietary covering that wraps around the stent to prevent plaque and emboli from dislodging and traveling to the brain during or after the procedure. This structural advancement offers a safer, minimally invasive alternative to traditional surgical methods, particularly for patients considered high-risk for carotid endarterectomy. With the FDA’s recent premarket approval, CGuard Prime is now set to enter the U.S. market as a first-line option for carotid revascularization.
Backed by robust data from the C-GUARDIANS pivotal trial, CGuard Prime achieved unprecedented outcomes: the lowest 30-day (0.95%) and 1-year (1.93%) major adverse event rates among all comparable carotid revascularization studies. This performance highlights its effectiveness in reducing procedural risks such as stroke, myocardial infarction, and death. The stent’s innovative design simplifies deployment while enhancing patient safety, making it especially suitable for symptomatic individuals or those with complex anatomical challenges. With over 65,000 units implanted globally and strong safety records, CGuard Prime brings both proven reliability and a major leap forward in interventional cardiology.
The FDA approval is more than a regulatory milestone—it is a critical inflection point for stroke care in the U.S. healthcare system. InspireMD is poised to initiate a rapid national launch of the CGuard Prime system, supported by new funding unlocked through its milestone-driven investment structure. The company’s growing portfolio of carotid innovations reflects a broader shift toward minimally invasive, neuroprotective technologies in vascular medicine. By combining clinical rigor, breakthrough engineering, and a clear path to commercialization, CGuard Prime stands to redefine how stroke prevention is approached and delivered.