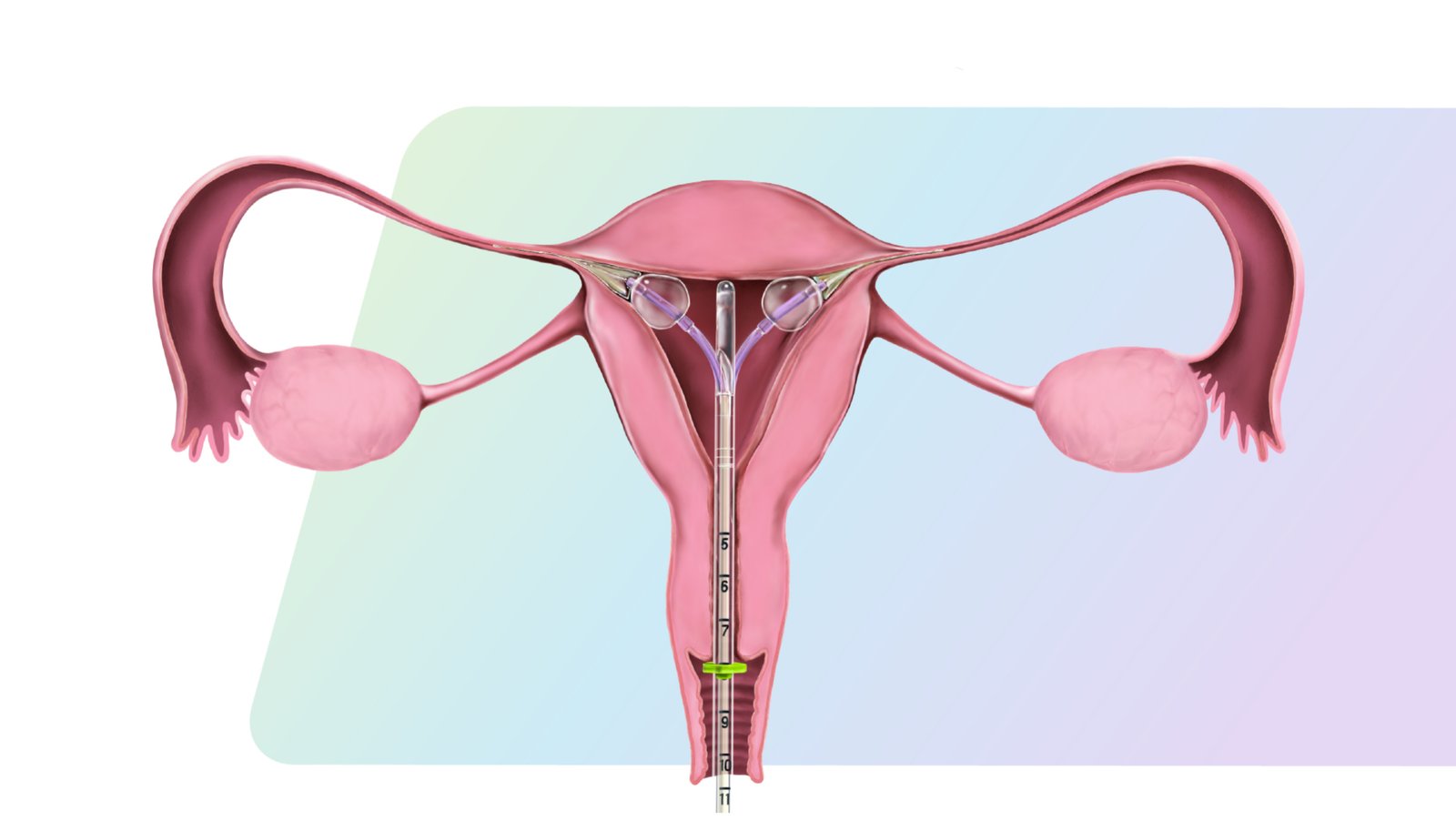
Femasys Inc. has achieved a pivotal breakthrough in women’s health with the European approval of FemBloc®, the world’s first non-surgical permanent birth control system. This dual-component technology—comprising a proprietary polymer and an in-office delivery system—has received CE mark certification under the EU Medical Device Regulation, allowing it to be marketed across the European Economic Area. Unlike traditional surgical sterilization, FemBloc is designed to be administered in a clinic setting without incisions, anesthesia, or the risks associated with surgery, offering a safer and more accessible solution for permanent contraception.
FemBloc works by delivering a biocompatible polymer into each fallopian tube, which triggers a natural tissue response to gradually form a permanent barrier to prevent fertilization. The system is intended for use in a brief, non-surgical procedure performed by a healthcare provider in an outpatient setting. Its patient-friendly design not only reduces clinical time and resource use but also allows women to return to normal activities immediately after the procedure. This innovative approach addresses longstanding concerns around surgical sterilization and opens new avenues for women seeking a permanent solution that is less invasive and more affordable.
With the CE mark now in place for both components of the FemBloc System, Femasys is positioned to launch in Europe, starting with Spain and expanding through strategic partnerships. This milestone validates years of development and clinical research and reflects growing global demand for alternatives to surgical contraception. As the company continues pivotal trials for U.S. FDA approval, the European rollout will play a crucial role in shaping the future of reproductive health by providing women with a groundbreaking, patient-centered option for permanent birth control.