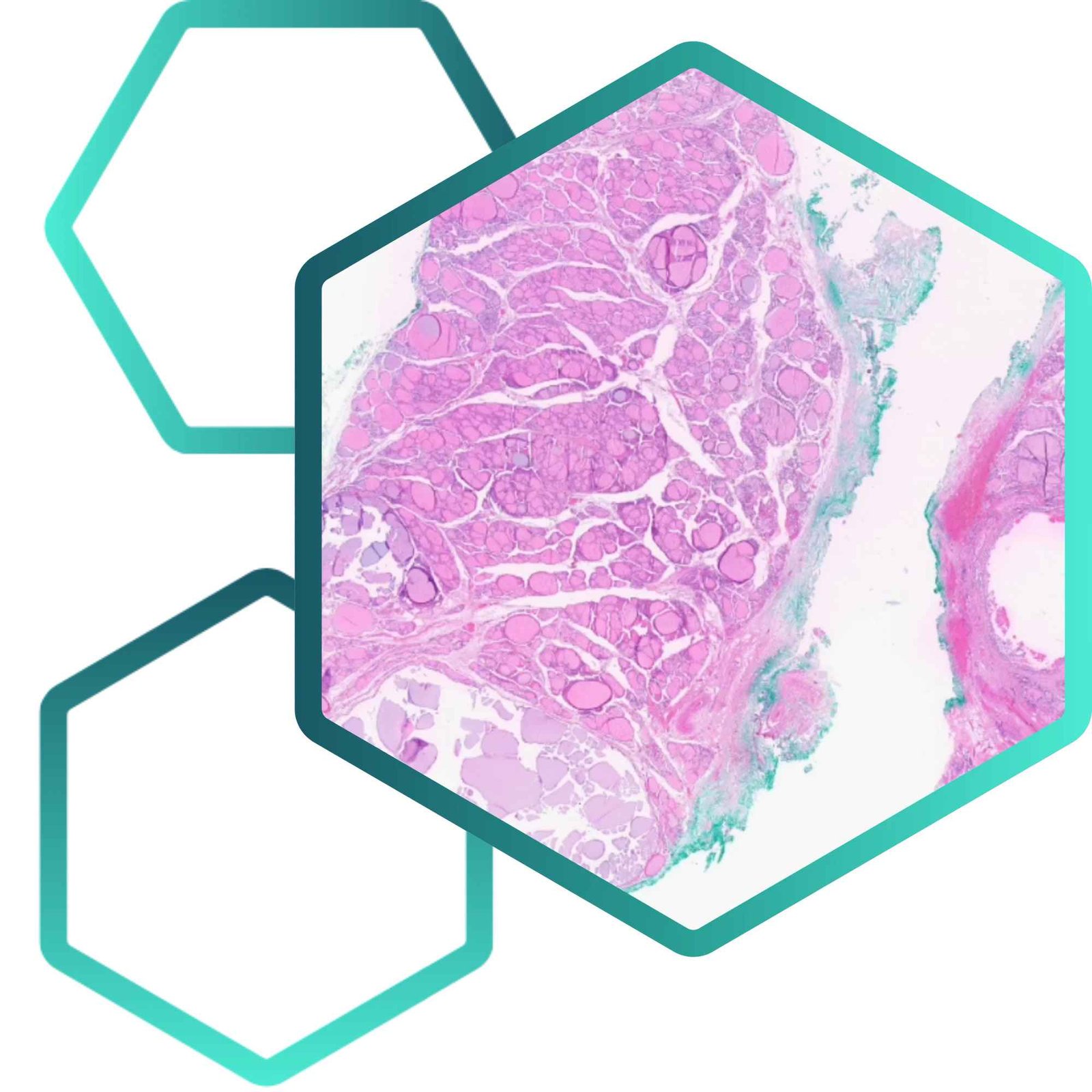
PathAI has achieved a major regulatory milestone with the FDA’s 510(k) clearance of its AISight® Dx platform for use in primary diagnosis, marking a significant step forward in the adoption of AI-powered digital pathology. AISight Dx is a cloud-native image management system that streamlines anatomic pathology workflows by integrating high-performance slide viewing, intelligent case management, and real-time collaboration tools. This clearance not only validates the platform’s clinical reliability but also signals growing trust in digital pathology as a safe and effective alternative to traditional microscope-based diagnostics.
One of the standout features of this FDA decision is the inclusion of a Predetermined Change Control Plan (PCCP), which allows PathAI to implement pre-approved updates—such as support for additional scanners, file formats, and display configurations—without the need for new 510(k) submissions. This accelerates the platform’s ability to evolve while maintaining regulatory compliance, giving labs access to cutting-edge features more rapidly. As a result, AISight Dx can adapt in real time to the fast-paced demands of modern pathology, helping institutions stay ahead in diagnostic innovation.
The clearance positions AISight Dx as a cornerstone solution for hospitals, academic centers, and pathology labs looking to modernize operations. By reducing manual workflow bottlenecks, improving turnaround times, and enabling remote collaboration, the platform supports more consistent and timely diagnoses—ultimately improving patient outcomes. With this latest advancement, PathAI reinforces its leadership in AI-driven healthcare solutions, delivering on its mission to transform pathology and set new standards in diagnostic accuracy and efficiency.