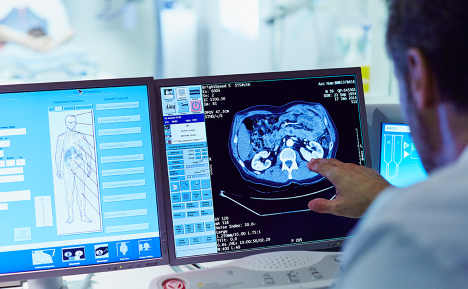
SimBioSys' latest FDA 510(k) clearance for TumorSight™ Viz 1.3 marks a significant advancement in the integration of artificial intelligence and spatial biophysics into breast cancer surgery. As the third regulatory approval for the TumorSight platform, this version enhances its ability to transform standard breast MRI scans into intuitive, 3D anatomical visualizations, enabling clinicians to make more precise and personalized surgical decisions. The innovation lies in its superior AI-driven segmentation and real-time tumor volume estimation, which allow for clearer preoperative planning and a better understanding of each patient's unique anatomical landscape.
The platform’s expanded functionality brings major advantages to surgical teams and patients alike. By reducing manual image-processing tasks through PACS connectivity, TumorSight Viz 1.3 streamlines workflows and delivers same-day results—facilitating more timely treatment discussions. The ability to visually map tumors in 3D improves accuracy in defining surgical margins, planning incisions, and optimizing cosmetic outcomes, which is increasingly important in today’s value-based care environment. Backed by validation across more than 1,600 cases from diverse institutions, the platform demonstrates high concordance with radiologist annotations and consistent performance across clinical settings.
As re-excision rates for breast cancer surgeries remain above 20%, the need for precision tools that can help avoid repeat procedures is clear. TumorSight Viz 1.3 not only enhances surgical clarity but also supports patient education and shared decision-making through visual aids that improve understanding and trust. With rising expectations around surgical personalization and cosmetic outcomes, SimBioSys’ innovation stands at the forefront of next-generation surgical planning. This latest clearance solidifies TumorSight Viz as a transformative solution in breast cancer care—one that empowers surgeons, supports clinical efficiency, and ultimately leads to better patient outcomes.