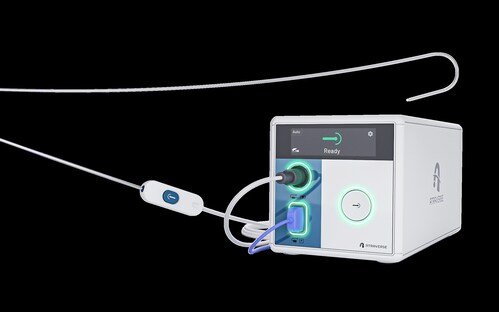
Atraverse Medical, a medical device company pioneering next-generation left-heart access technology, has received 510(k) clearance from the U.S. FDA for its fully integrated HOTWIRE Transseptal Access System.
The novel system includes the HOTWIRE RF Generator (August 2025) featuring impedance-guided shutoff after transseptal crossing — mitigating the risk of uncontrolled energy delivery after accessing the left atrium — along with an ability to activate energy within the sterile field, giving more precise control to clinicians. The FDA 510(k) cleared HOTWIRE RF Guidewire (May 2024) has been utilized successfully in nearly 2,000 clinical procedures to date, and these new features are designed to enhance procedural control and workflow efficiency with an end-to-end, zero-exchange, sheath-agnostic solution.
The fully integrated HOTWIRE Transseptal Access System includes:
HOTWIRE RF Generator: The first and only left-heart access system with impedance-guided technology that halts energy delivery upon transseptal crossing, minimizing unnecessary RF exposure in the left atrium, and enabling user-controlled energy activation in the sterile field.
HOTWIRE RF Guidewire: A zero-exchange guidewire with universal sheath compatibility, with a proprietary tip architecture that enhances echocardiographic visualization by 25 per cent, and a reinforced core wire and polymer jacket with twice the rail stiffness of leading competitors for controlled advancement of large bore sheaths
Dr Devi Nair, Director of Cardiac Electrophysiology at St. Bernard's Heart and Vascular Center and world-renowned key-opinion leader, conducted the first clinical cases with the full HOTWIRE system and will be presenting data at the upcoming AF Symposium on her experience.