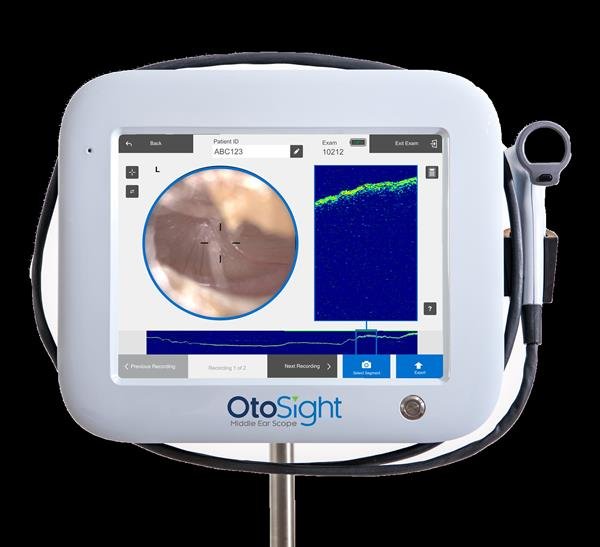
PhotoniCare’s OtoSight™ Middle Ear Scope represents a significant technological advancement in pediatric otolaryngology. Utilizing Optical Coherence Tomography (OCT), OtoSight enables clinicians to see through the eardrum to detect and characterize middle ear fluid with high accuracy—even in the presence of wax, which often obscures traditional visual tools. This breakthrough allows for a more precise differentiation between bacterial and non-bacterial infections, which is not possible with standard otoscopy. OtoSight is the first and only FDA-cleared device for imaging the middle ear, setting a new benchmark in diagnostic innovation with unique CPT codes that support billing and reimbursement.
The OtoSight device is designed for real-world clinical environments, including pediatric practices, family medicine, and ENT clinics. In the OTO-MATIC randomized controlled trial, use of the device led to a 50% reduction in antibiotic prescriptions compared to standard care. This improved accuracy also led to fewer instances of combination therapy, promoting more targeted and effective treatment strategies. By equipping clinicians with clearer diagnostic insight, OtoSight enhances decision-making, reduces the risk of overtreatment, and contributes to a more efficient workflow in primary care settings.
OtoSight addresses a critical gap in pediatric care—accurate diagnosis of ear infections, which are a leading cause of antibiotic use and surgery in children. With up to 50% misdiagnosis rates using traditional tools, many children face repeated infections, unnecessary medications, and invasive procedures like ear tube surgery. The device not only improves outcomes for patients but also supports global public health efforts around antibiotic stewardship. As validated by ongoing clinical trials and peer-reviewed publications, OtoSight is set to become an essential tool in frontline pediatric care, transforming how ear infections are managed and treated.
PhotoniCare’s OtoSight™ Middle Ear Scope uses Optical Coherence Tomography (OCT) to visualize fluid behind the eardrum with high precision—surpassing traditional otoscopy by enabling accurate diagnosis even in the presence of earwax.
In a large real-world randomized controlled trial, OtoSight reduced antibiotic prescriptions by 50% and improved single-therapy treatment decisions, addressing overuse of antibiotics and high misdiagnosis rates in pediatric ear infections.
OtoSight sets a new standard in pediatric ear care by combining diagnostic accuracy with clinical efficiency, helping reduce unnecessary treatments and surgeries while supporting better long-term outcomes for children.