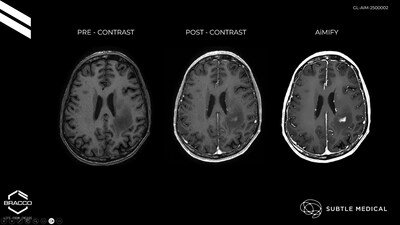
AiMIFY™, developed through the collaboration between Bracco Imaging and Subtle Medical, represents a breakthrough in AI-powered medical imaging. The software uses advanced deep learning algorithms to enhance contrast in brain MRI scans—achieving up to double the visual contrast typically produced by standard gadolinium-based contrast agents (GBCAs). This innovation enables radiologists to detect subtle abnormalities, such as small or poorly enhanced lesions, without requiring changes to imaging protocols or additional contrast dosage, making it a seamless upgrade to existing diagnostic workflows.
In clinical practice, AiMIFY™ is designed to integrate effortlessly into MRI systems, providing enhanced image clarity in real time. Its broad compatibility across scanner vendors, imaging sequences, and patient types ensures widespread utility and consistent performance. This added layer of diagnostic precision is especially valuable in early disease detection and complex neurological assessments, helping physicians make faster, more confident decisions while minimizing patient risk from excessive contrast use.
The CE Mark approval under the European Medical Device Regulation marks a significant step forward for AI in radiology. It affirms AiMIFY™’s compliance with rigorous EU standards and positions it for broad adoption across European healthcare systems. As Bracco and Subtle Medical prepare for commercial rollout, AiMIFY™ stands to play a key role in advancing precision diagnostics, enhancing patient outcomes, and exemplifying how AI can elevate existing clinical tools without disrupting standard care practices.
MedTech Spectrum's Summary
Enhances MRI Precision with AI: AiMIFY™ significantly improves brain MRI contrast using deep learning, aiding in earlier and more accurate lesion detection without requiring changes to existing imaging protocols.
Seamless Clinical Integration: The software is compatible across various scanners and workflows, offering immediate diagnostic value while maintaining standard practices and minimizing contrast agent usage.
Validated for European Expansion: With CE Mark approval, AiMIFY™ is set for commercial rollout across Europe—marking a major milestone in the clinical adoption of AI-powered imaging tools to support precision diagnostics.