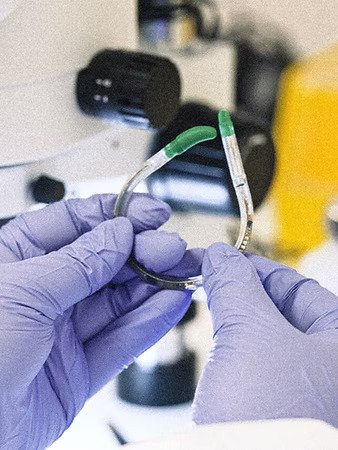
Bright Uro has made a significant breakthrough in urodynamic testing with its Glean™ Urodynamics System, the first and only FDA-cleared wireless, catheter-free solution for assessing lower urinary tract dysfunction (LUTD). The system, designed for ambulatory use, enables clinicians to collect accurate bladder function data in a physiological setting without the interference of traditional catheters. The recently published MUSE (Modern Urodynamics System Efficacy) feasibility study confirmed that the Glean sensor is safe, well-tolerated, and easy to place and remove, with all 32 patients able to void naturally while using the device—something often not possible with conventional methods.
Traditional urodynamic tests often produce results that don’t align with patients’ actual symptoms due to their invasive and non-physiological nature. Glean resolves this by using a small intravesical sensor to wirelessly record bladder pressure without disrupting natural urinary function. This innovation allows for real-world, in-clinic data collection and has been rated highly by both patients and clinicians. The MUSE trial showed high success in sensor placement and removal, with no serious adverse events, highlighting the system’s safety and user-friendliness. The technology also offers a practical advantage for health systems—reducing capital and staffing burdens, thus expanding access to care.
The positive findings from the MUSE study establish a strong case for Glean™ as a next-generation diagnostic tool in urology. By enabling more physiological, non-invasive testing, Bright Uro’s solution improves diagnostic accuracy, increases patient comfort, and lowers barriers to access across diverse care settings. As more clinical data emerges, the Glean Urodynamics System may soon become a new standard for evaluating bladder function—replacing outdated approaches with a streamlined, scalable, and patient-centered alternative.
MedTech Spectrum's Summary
First-of-Its-Kind Technology: Glean™ is the first FDA-cleared, wireless, catheter-free urodynamics system, enabling more natural and accurate testing of lower urinary tract function.
Improved Patient Experience: The system was proven safe, easy to use, and well-tolerated in the MUSE study, with all patients able to void normally—addressing a major limitation of traditional catheter-based testing.
Expanded Access and Efficiency: Glean™ reduces clinical complexity and cost, allowing broader adoption across healthcare sites, which can streamline diagnostics and improve access for patients needing urodynamic evaluations.