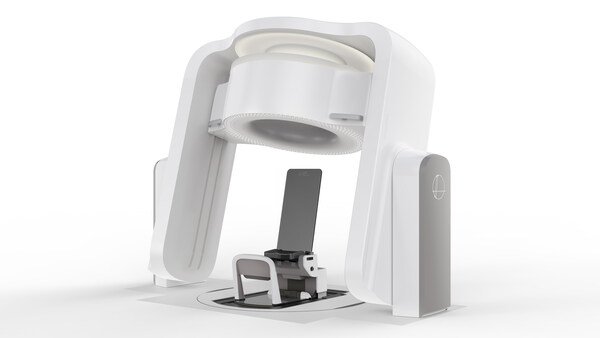
Leo Cancer Care has reached a key regulatory milestone with its upright particle therapy solution, Marie®, now officially 510(k) pending with the U.S. FDA. This submission marks a significant step toward clinical adoption in the United States, bringing Leo Cancer Care closer to transforming how cancer patients receive radiotherapy.
Marie® is a pioneering system that merges Leo’s upright patient positioning platform with an integrated fan beam CT scanner. The solution is designed to be used with any fixed particle beam, enabling providers to eliminate the need for large, costly rotating gantries. This not only reduces construction and shielding requirements but also dramatically lowers the footprint and cost of particle therapy centers.
Unlike conventional radiotherapy, which treats patients lying down, Leo’s approach positions the patient upright, rotating them instead of the machine. This design aims to improve patient comfort, support better treatment accuracy, and make advanced therapies like proton and carbon ion therapy more widely accessible.
“Years of development have led to this moment,” said Stephen Towe, CEO of Leo Cancer Care. “Reaching the 510(k) pending milestone is a testament to the vision and hard work of our team and partners. We believe Marie will bring meaningful change to particle therapy by making it more scalable and patient-centered.”
Several U.S. facilities have already begun preparing for Marie’s implementation and are eagerly awaiting FDA clearance. The company expects the first patient treatment using Marie to take place later this year — a historic leap for upright cancer care worldwide.
Medtech Spectrum Summary
FDA Progress: Leo Cancer Care’s Marie is now 510(k) pending, signaling regulatory advancement in upright radiotherapy delivery.
Disruptive Design: Replaces rotating gantries with upright patient positioning and fixed beams, shrinking system size and cost.
Market Readiness: U.S. centers preparing for first-in-the-world upright particle therapy treatment once clearance is granted.